Your How to calculate natural abundance images are ready. How to calculate natural abundance are a topic that is being searched for and liked by netizens today. You can Get the How to calculate natural abundance files here. Download all royalty-free vectors.
If you’re looking for how to calculate natural abundance pictures information connected with to the how to calculate natural abundance keyword, you have pay a visit to the ideal blog. Our website always provides you with hints for seeking the maximum quality video and image content, please kindly hunt and locate more informative video articles and images that fit your interests.
How To Calculate Natural Abundance. Percentage abundance usually can be divided by 100 to get fractional abundance. How Do You Calculate the Natural Abundance of Isotopes. When it comes to the actual calculation its easier to use decimal abundances which are simply percent abundances divided by 100. 3 Calculate atoms of Ne in 06219 mol.
 Isotopes And Atomic Mass 2 Of 3 Youtube From youtube.com
Isotopes And Atomic Mass 2 Of 3 Youtube From youtube.com
Identifying isotopes and ions. Note that this equation is limited to two isotopes. Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon. Hence the percent abundance of three isotopes are given by. This is the currently selected item. Calculate the atomic weight of boron.
Calculate the atomic weight of boron.
X 1 x 1. Abundance of 11 5 B 1000 - abundance of 10 5 B abundance of 11 5 B 1000 - 200 abundance of 11 5 B 800. This means that their respective decimal abundance must add up to give 1. As a percent the equation would be. Since boron only has two isotopes the abundance of one must be 1000 - the abundance of the other. Percentage abundance usually can be divided by 100 to get fractional abundance.
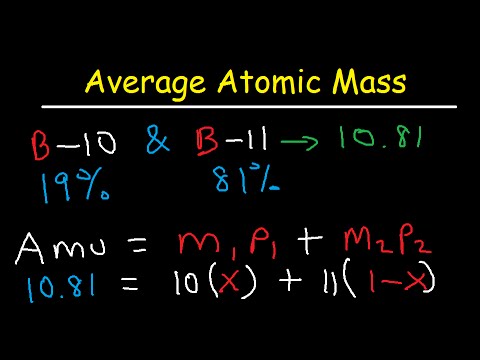 Source: youtube.com
Source: youtube.com
AM is the average atomic mass. Preparing to study chemistry. The formula to find the percent abundance of an element with two isotopes is as follows. 3 Calculate atoms of Ne in 06219 mol. Atomic mass 34969 amu and.
 Source: clutchprep.com
Source: clutchprep.com
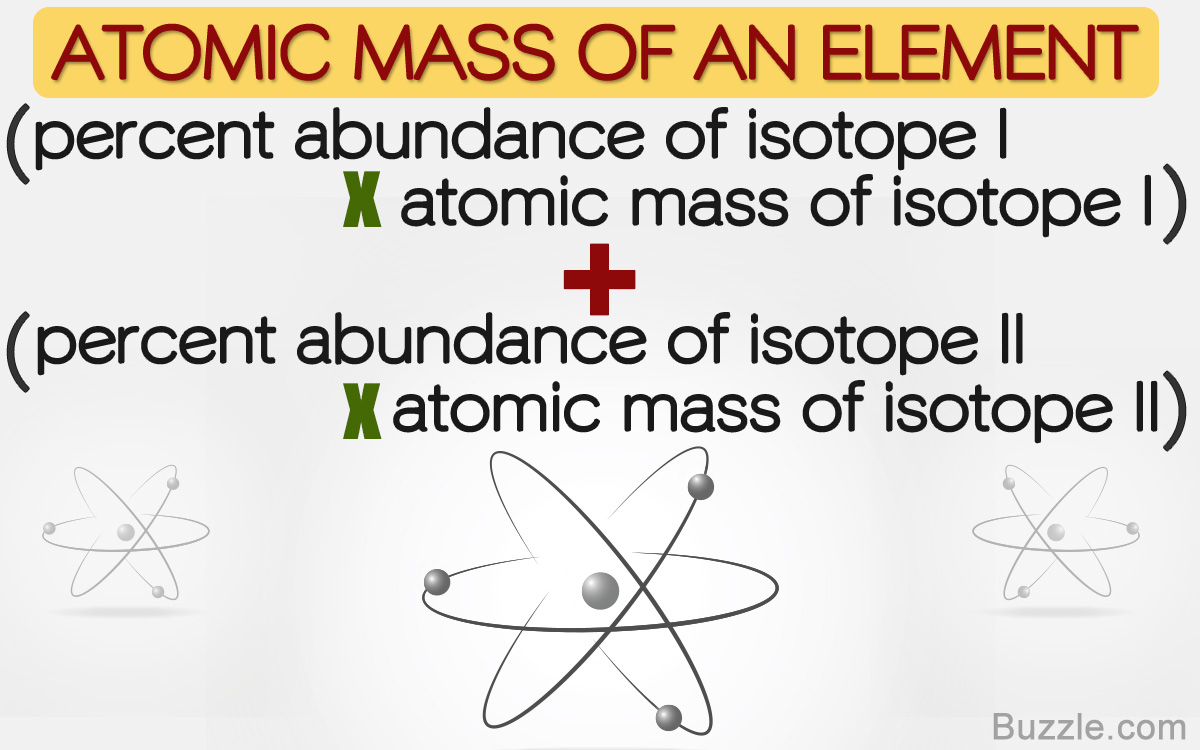
To calculate the percent abundance of each isotope in a sample of an element chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100. 4192 chlorine-35 07577 34969 2650 amu chlorine-37 02423 36966 8957 amu average. Mass number The mass number is another common term that generally refers to the sum of protons and neutrons in the nucleus of an atom. If you set the equation as a decimal this means the abundance would be equal to 1. 1 Calculate the percent abundance of the two isotopes.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Note that this equation is limited to two isotopes. When it comes to the actual calculation its easier to use decimal abundances which are simply percent abundances divided by 100. Atomic number mass number and isotopes. The mole and Avogadros number. 4345 chromium-52 8379 chromium-53 950 chromium-54 2365.
 Source: youtube.com
Source: youtube.com
Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon. So you know that copper has two naturally occurring isotopes copper-63 and copper-65. X 1 x 1. Atomic mass 34969 amu and. List the known and unknown quantities and plan the problem.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
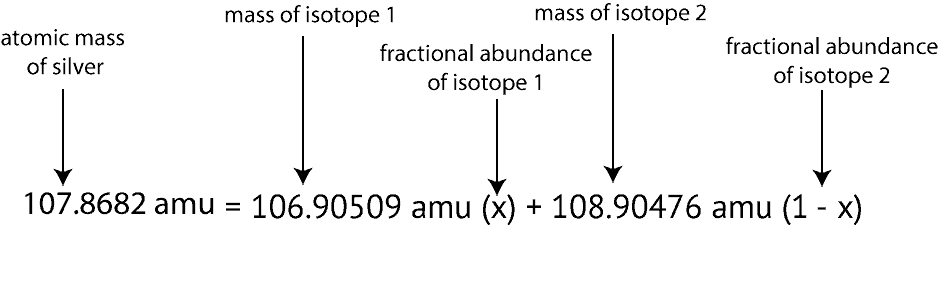
So you know that copper has two naturally occurring isotopes copper-63 and copper-65. So you know that copper has two naturally occurring isotopes copper-63 and copper-65. Subsequently question is how do you find the average atomic mass of an isotope. X 1 x 1. 4345 chromium-52 8379 chromium-53 950 chromium-54 2365.
 Source: clutchprep.com
Source: clutchprep.com
If you look in the periodic table you will be able to check that our answer is correct. 3 Calculate atoms of Ne in 06219 mol. 1255 g 2018 gmol 06219 mol. To find the average atomic mass for Carbon. B refers to the first isotopes atomic mass and c refers to the second isotopes atomic mass.
 Source: youtube.com
Source: youtube.com
When it comes to the actual calculation its easier to use decimal abundances which are simply percent abundances divided by 100. X 1 x 1. Note that this equation is limited to two isotopes. Example 1 The natural abundance for boron isotopes is. So you know that copper has two naturally occurring isotopes copper-63 and copper-65.
 Source: sciencestruck.com
Source: sciencestruck.com
The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by mz 2 with a relative intensity of 31. The following formula is used to calculate the percent abundance of an isotope. Average Atomic Mass 120000 9890 130033 00110 12011 amu. How do you calculate the natural abundance of two isotopes. Hence the percent abundance of three isotopes are given by.
 Source: youtube.com
Source: youtube.com
In chemistry natural abundance refers to the abundance of isotopes of a chemical element that is naturally found on a planet. 4345 chromium-52 8379 chromium-53 950 chromium-54 2365. This is the currently selected item. To perform the calculation one must use the following formula. To calculate the percent abundance of each isotope in a sample of an element chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
 Source: youtube.com
Source: youtube.com
Periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeatomic mass of chromium is 51996 amu chromium-. In chemistry natural abundance refers to the abundance of isotopes of a chemical element that is naturally found on a planet. So we get the abundance of 16O is 09976 and the abundance of 18O is 099963 09976 000203. Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon. The mass spectrum of CH 3 Cl Figure 21 clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom.

Percentage abundance is always reported as a percentage and it is calculated as. Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon. X 1 x 1. As a percent the equation would be. Identifying isotopes and ions.
 Source: kentchemistry.com
Source: kentchemistry.com
To find the average atomic mass for Carbon. To perform the calculation one must use the following formula. Mass number The mass number is another common term that generally refers to the sum of protons and neutrons in the nucleus of an atom. Natural abundance quite frequently used in chemistry refers to how abundantly a specific isotope of a given element is found on the Earth naturally. The mass spectrum of CH 3 Cl Figure 21 clearly shows two peaks with the isotope distribution pattern for an ion with a single chlorine atom.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Calculate the atomic weight of boron. The table below shows the exact mass of each isotope isotopic mass and the percent abundance sometimes called fractional abundance for the primary isotopes of Carbon. X 100-x 100 where the 100 designates the total percent in nature. X 100-x 100 where the 100 designates the total percent in nature. Since boron only has two isotopes the abundance of one must be 1000 - the abundance of the other.
 Source: youtube.com
Source: youtube.com
The mole and Avogadros number. Percentage abundance usually can be divided by 100 to get fractional abundance. 199 10 B 10013 amu and 801 11 B 11009amu. Where A is the percent abundance. Example 1 The natural abundance for boron isotopes is.
 Source: youtube.com
Source: youtube.com
It uses bromine-79 and bromine-81 as an exampleMy Website. It uses bromine-79 and bromine-81 as an exampleMy Website. Average Atomic Mass 120000 9890 130033 00110 12011 amu. The equation would then become. IM is the mass of the isotope.
 Source: youtube.com
Source: youtube.com
4345 chromium-52 8379 chromium-53 950 chromium-54 2365. To find the average atomic mass for Carbon. Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon. This means that their respective decimal abundance must add up to give 1. Periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeatomic mass of chromium is 51996 amu chromium-.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
X 1 x 1. 1 Calculate the percent abundance of the two isotopes. Note that this equation is limited to two isotopes. The relative abundance for a specific ion in the sample can be calculated by dividing by the number of ions with a particular m z mz mz ratio by the total number of ions detected. To calculate the percent abundance of each isotope in a sample of an element chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
 Source: toppr.com
Source: toppr.com
Abundance of 11 5 B 1000 - abundance of 10 5 B abundance of 11 5 B 1000 - 200 abundance of 11 5 B 800. So you know that copper has two naturally occurring isotopes copper-63 and copper-65. Atomic mass 34969 amu and. As a percent the equation would be. Calculate the number of Ne-22 atoms in a 1255 g sample of naturally occuring neon.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate natural abundance by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






