Your How to find the natural abundance of an isotope images are ready. How to find the natural abundance of an isotope are a topic that is being searched for and liked by netizens today. You can Find and Download the How to find the natural abundance of an isotope files here. Get all royalty-free photos and vectors.
If you’re looking for how to find the natural abundance of an isotope pictures information connected with to the how to find the natural abundance of an isotope keyword, you have visit the ideal blog. Our site frequently gives you suggestions for viewing the highest quality video and image content, please kindly search and find more informative video articles and graphics that match your interests.
How To Find The Natural Abundance Of An Isotope. Percentage abundance usually can be divided by 100 to get fractional abundance. Let x be the unknown abundance of 16O and other isotope abundance of 18O be 099963-x. Carbon 6 C 12011 isotope abundance mass amu. It is actually a weighted mass of the elements isotopes if any and their relative abundance.
 Solve For Isotopic Abundance Chemistnate From chemistnate.com
Solve For Isotopic Abundance Chemistnate From chemistnate.com
Regarding this how do you calculate percent abundance. Example 1 The natural abundance for boron. For this example the average atomic mass is found to be. B refers to the first isotopes atomic mass and c refers to the second isotopes atomic mass. Now modifying equation i we get 15995 x 16999 000037 17999 099963 x 159994. The mass of the longest lived isotope is given for elements without a stable nuclide.
Beside above which isotope of boron is most abundant.
Remember to convert. This table lists the mass and percent natural abundance for the stable nuclides. It uses bromine-79 and bromine-81 as an exampleMy Website. For example if we take a weighted average for the isotopes of Carbon we get an average atomic mass of 12011 amu. C There are equal amounts. The formula to find the percent abundance of an element with two isotopes is as follows.
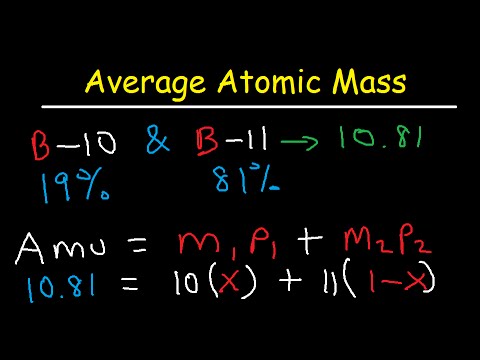 Source: youtube.com
Source: youtube.com
Average mass of an element atomic mass of isotope I X percent abundance of isotope I100 atomic mass of isotope II X percent abundance of isotope II100. Atoms of the same element with different numbers of neutrons. Calculate the atomic mass of gallium. E none of the above. Bx 1-x c a and the unknown abundance is the x.
 Source: youtube.com
Source: youtube.com
06219 mol times 6022 x 10 23 mol-1 3745 x. Firstly we have the abundance of one isotope which is 00037. Finally calculate the percent abundance using the formula above. The formula to find the percent abundance of an element with two isotopes is as follows. Let x be the unknown abundance of 16O and other isotope abundance of 18O be 099963-x.
 Source: youtube.com
Source: youtube.com
For this example the average atomic mass is found to be. I am using y to avoid confusion with the x used by chemistry123 and so that I can use X as a multiplication sign without confusion. Used to account for the natural abundances of isotopes. With ypercentage of 109Ag expressed as a decimal. Multiply this value by the atomic mass of that isotope.
 Source: youtube.com
Source: youtube.com
Percent abundance100 x amu atomic weight gmol. Use algebra to solve for x. Using the average mass from the periodic table calculate the abundance of each isotope. The equation continues on based on the number of isotopes in the problem. Average mass of an element atomic mass of isotope I X percent abundance of isotope I100 atomic mass of isotope II X percent abundance of isotope II100.
 Source: youtube.com
Source: youtube.com
06219 mol times 6022 x 10 23 mol-1 3745 x. 1255 g 2018 gmol 06219 mol. Now modifying equation i we get 15995 x 16999 000037 17999 099963 x 159994. So the abundances of the other two remaining isotopes is 1-000037 099963. M1x M21-x ME Example problem.
 Source: clutchprep.com
Source: clutchprep.com
Gallium has two naturally occurring isotopes. Number of atoms of an isotope divided by the total number of atoms of all isotopes of that element multiplied by 100. Nuclides marked with an asterisk in the abundance column indicate that it is not present in nature or that a meaningful natural abundance cannot be given. So the abundances of the other two remaining isotopes is 1-000037 099963. Percentage abundance usually can be divided by 100 to get fractional abundance.
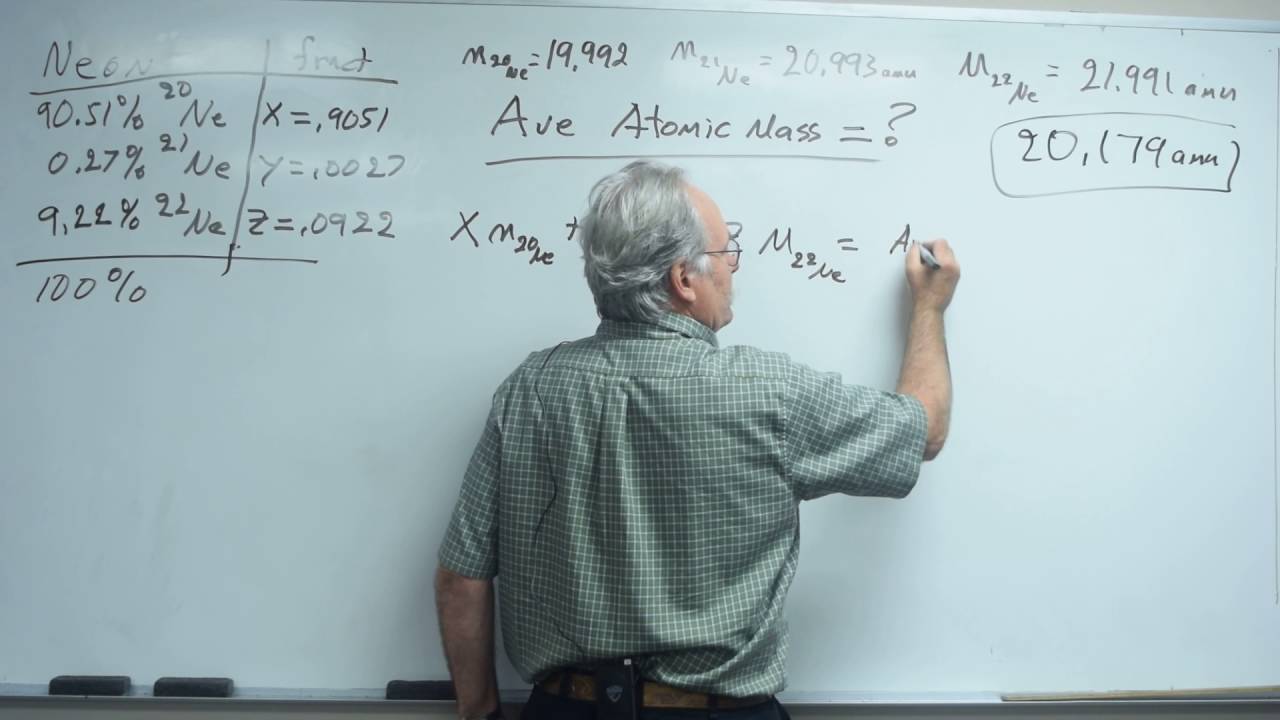 Source: chem.libretexts.org
Source: chem.libretexts.org
I am using y to avoid confusion with the x used by chemistry123 and so that I can use X as a multiplication sign without confusion. Percent abundance100 x amu atomic weight gmol. B refers to the first isotopes atomic mass and c refers to the second isotopes atomic mass. The mass of antimony-121 is 120904 amu and the mass of antimony-123 is 122904 amu. C There are equal amounts.
 Source: youtube.com
Source: youtube.com
Calculate the atomic mass of gallium. Percentage abundance is always reported as a percentage and it is calculated as. The atomic weight of an element is the relative atomic mass of that element. Using the average mass from the periodic table calculate the abundance of each isotope. If the masses of one isotope of nitrogen nitrogen-14 is 14003 amu and another isotope nitrogen-15 is 15000 amu find the relative abundance of the isotopes.
 Source: youtube.com
Source: youtube.com
If the masses of one isotope of nitrogen nitrogen-14 is 14003 amu and another isotope nitrogen-15 is 15000 amu find the relative abundance of the isotopes. 1 Calculate the percent abundance of the two isotopes. To get an accurate answer each isotopes atomic mass and the elements specific atomic mass are necessary. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. Nuclides marked with an asterisk in the abundance column indicate that it is not present in nature or that a meaningful natural abundance cannot be given.
 Source: youtube.com
Source: youtube.com
To perform the calculation one must use the following formula. Percentage abundance usually can be divided by 100 to get fractional abundance. Finally calculate the percent abundance using the formula above. B refers to the first isotopes atomic mass and c refers to the second isotopes atomic mass. 06219 mol times 6022 x 10 23 mol-1 3745 x.
 Source: toppr.com
Source: toppr.com
Calculate the atomic mass of gallium. Use the following formula for relative abundance chemistry problems. 1999 1-x 2199 x 2018 x 0095 this is the Ne-22 2 Calculate moles of Ne in 1255 g. Unit used to express the masses of atoms andor subatomic part. Multiply this value by the atomic mass of that isotope.
 Source: chemistnate.com
Source: chemistnate.com
10787 10890y 10690 1-y 10787 10890y 10690 - 10690y. Finding the Average Atomic Mass of an Element with Isotopes Multiply each isotopes mass by its percent abundance. Example 1 The natural abundance for boron. Gallium has two naturally occurring isotopes. Used to account for the natural abundances of isotopes.
 Source: clutchprep.com
Source: clutchprep.com
It uses bromine-79 and bromine-81 as an exampleMy Website. To get an accurate answer each isotopes atomic mass and the elements specific atomic mass are necessary. Multiply this value by the atomic mass of that isotope. Atomic mass mass 1 1 mass 2 2. The atomic weight of an element is the relative atomic mass of that element.
 Source: youtube.com
Source: youtube.com
The mass of the longest lived isotope is given for elements without a stable nuclide. Let x be the unknown abundance of 16O and other isotope abundance of 18O be 099963-x. If the masses of one isotope of nitrogen nitrogen-14 is 14003 amu and another isotope nitrogen-15 is 15000 amu find the relative abundance of the isotopes. To get an accurate answer each isotopes atomic mass and the elements specific atomic mass are necessary. To perform the calculation one must use the following formula.
 Source: youtube.com
Source: youtube.com
Regarding this how do you calculate percent abundance. Average mass of an element atomic mass of isotope I X percent abundance of isotope I100 atomic mass of isotope II X percent abundance of isotope II100. Multiply this value by the atomic mass of that isotope. Unit used to express the masses of atoms andor subatomic part. Next determine the mass of the isotope.
 Source: youtube.com
Source: youtube.com
In physics natural abundance NA refers to the abundance of isotopes of a chemical element as naturally found on a planet. 1 Calculate the percent abundance of the two isotopes. Percentage abundance usually can be divided by 100 to get fractional abundance. Next determine the mass of the isotope. For this example the isotope mass is found to be 200.
 Source: youtube.com
Source: youtube.com
Example problemIf the masses of. Finally calculate the percent abundance using the formula above. In physics natural abundance NA refers to the abundance of isotopes of a chemical element as naturally found on a planet. Let x be the unknown abundance of 16O and other isotope abundance of 18O be 099963-x. Add together for each isotope to get the average atomic mass.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
C There are equal amounts. If the masses of one isotope of nitrogen nitrogen-14 is 14003 amu and another isotope nitrogen-15 is 15000 amu find the relative abundance of the isotopes. With ypercentage of 109Ag expressed as a decimal. Next determine the mass of the isotope. Gallium has two naturally occurring isotopes.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find the natural abundance of an isotope by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






