Your Natural gas combustion equation images are ready. Natural gas combustion equation are a topic that is being searched for and liked by netizens today. You can Find and Download the Natural gas combustion equation files here. Download all royalty-free images.
If you’re looking for natural gas combustion equation images information related to the natural gas combustion equation keyword, you have pay a visit to the ideal blog. Our website always provides you with hints for seeing the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that fit your interests.
Natural Gas Combustion Equation. Major groups of nitric compounds are. Natural gas is the cleanest burning fossil fuel. Perfect combustion of natural gas Methane CH 4 produces only CO2 and water vapor The equation for the combustion of natural gas is. How would we know without actual data.
 Chapter 11 Combustion Updated 5 31 10 From ohio.edu
Chapter 11 Combustion Updated 5 31 10 From ohio.edu
CH 4 2O 2 CO 2 2H 2 O. A reaction with the dioxygen O2 in the air when the mixture of air and methane is heated with an electrical spark typically or with the flame of a match. CH_4 O_2 to CO_2 H_2O But the reaction is. Natural gas consists of a high percentage of methane generally above. Respect to natural gas combustion. COMBUSTION AND FUELS FUEL NITROGEN IN GAS Natural gas practically doesnt have fuel nitrogen.
CH_4 O_2 to CO_2 H_2O But the reaction is.
Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely. Methane is CH4 and when burnt in oxygen air it produces heat and CO2 and water. H comb of methane from these values. The combustion reaction of methane is carried out even with an insufficient supply of oxygen. Commonly called burning a chemical reaction where a substance reacts quickly with oxygen gas realeasing energy in the form of light and heat. Burning methane releases only carbon dioxide and water.
 Source: ohio.edu
Source: ohio.edu
It is mainly used to generate industrial and utility electric power produce industrial process steam and heat and heat residential and commercial space. Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely. Answer 1 of 3. When natural gas burns a high-temperature blue flame is produced and complete combustion takes place. Perfect combustion of natural gas Methane CH 4 produces only CO2 and water vapor The equation for the combustion of natural gas is.
 Source: web.fscj.edu
Source: web.fscj.edu
A complete combustion is a process burning all the carbon C to CO 2 all the hydrogen H to H 2 O and all the sulphur S to SO 2. Complete Combustion Of Propane C3h8 Balanced Equation You. Not exactly the byproducts but simply the products of the reaction on a chemical point of view. For residential and commercial space heating. Perfect combustion of natural gas Methane CH 4 produces only CO2 and water vapor The equation for the combustion of natural gas is.
 Source: researchgate.net
Source: researchgate.net
Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely. CH4 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Commonly called burning a chemical reaction where a substance reacts quickly with oxygen gas realeasing energy in the form of light and heat. Modern gas turbines operate at high temperatures and use component designs and materials at ABSTRACT the forefront of technology but these are more susceptible to It is important that gas turbines used in Oil Gas applications can burn a wide variety of fuels with the minimum impact on the environment or economics. Natural gas consists of a high percentage of methane generally above.
 Source: youtube.com
Source: youtube.com
Calculate the flue gas composition both dry and wet basis leaving a boiler. Perfect combustion of natural gas Methane CH 4 produces only CO2 and water vapor The equation for the combustion of natural gas is. Stoichiometric or Theoretical Combustion is the ideal combustion process where fuel is burned completely. Not exactly the byproducts but simply the products of the reaction on a chemical point of view. The reaction is as follows.
 Source: ohio.edu
Source: ohio.edu
Writing And Balancing Combustion Reactions Lesson Transcript Study Com. A complete combustion is a process burning all the carbon C to CO 2 all the hydrogen H to H 2 O and all the sulphur S to SO 2. Question 31 2 In The Combustion Of Natural Gas Chegg Com. It is used mainly for industrial process steam and heat production. Calculate the heat of combustion of ethane as described in the equation C2H6g 3½O2g 2CO2g 3H2Ol given the heats of formation of ethane gas carbon dioxide gas and water liquid are 847 kJ mol-1 -3935 kJ mol-1 and 2858 kJ mol-1 respectively.
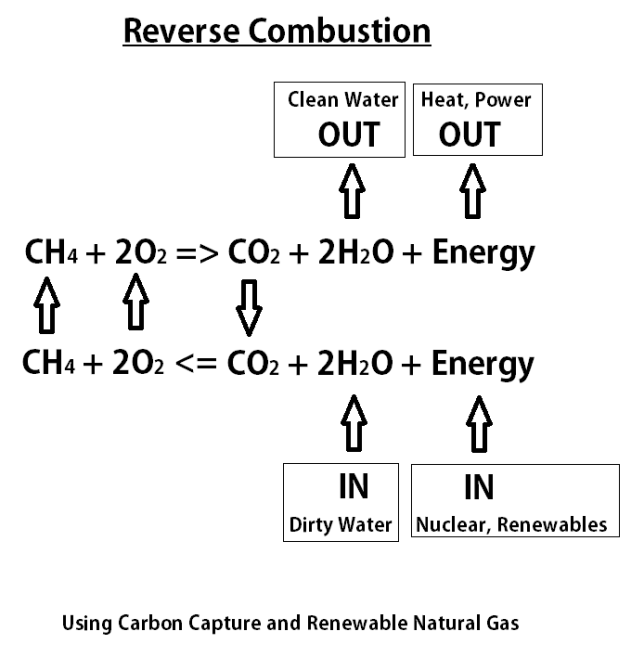 Source: edwardtdodge.com
Source: edwardtdodge.com
Question 31 2 In The Combustion Of Natural Gas Chegg Com. When we say that methane is combustible it means that it is possible to burn it. When this reaction takes place the result is carbon dioxide CO 2 water H 2 O and a great deal of energy. Major groups of nitric compounds are. A complete combustion is a process burning all the carbon C to CO 2 all the hydrogen H to H 2 O and all the sulphur S to SO 2.
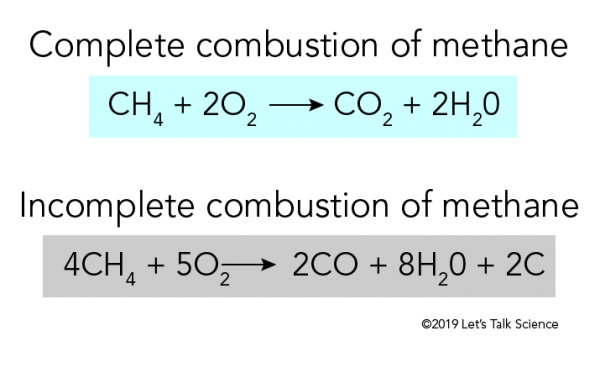 Source: letstalkscience.ca
Source: letstalkscience.ca
CH4 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Primary Chamber Outlet Gas Stream Plus Clean Ash HHV LHV Sensible Item lbhr MMBtuh MMBtuh Heat MMBtuh Total gas 102786 4453 Total solids 33200 1384 Rad. Respect to natural gas combustion. The reaction is as follows. How would we know without actual data.
 Source: ohio.edu
Source: ohio.edu
Calculate the flue gas composition both dry and wet basis leaving a boiler. Major groups of nitric compounds are. Calculate the heat of combustion of ethane as described in the equation C2H6g 3½O2g 2CO2g 3H2Ol given the heats of formation of ethane gas carbon dioxide gas and water liquid are 847 kJ mol-1 -3935 kJ mol-1 and 2858 kJ mol-1 respectively. The reaction is as follows. Governing EquationsNatural Gas and Biogas Combustion Modeling The equations used for natural gas biogas and natural gasbiogas mixture fuels combustion modeling 3 are based on the equations of conservation of.
 Source: ohio.edu
Source: ohio.edu
When we say that methane is combustible it means that it is possible to burn it. CH_4 O_2 to CO_2 H_2O But the reaction is. Burning methane releases only carbon dioxide and water. When natural gas burns a high-temperature blue flame is produced and complete combustion takes place. COMBUSTION AND FUELS FUEL NITROGEN IN LIQUID FUELS Crude oil has fuel nitrogen in the range of 001 do 03 wt.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The balanced reaction is. To calculate the Carbon Dioxide - CO 2 - emission from a fuel the carbon content of the fuel must be multiplied with the ratio of molecular weight of CO 2 44 to the molecular weight of Carbon 12. Introduction - Up to this point the heat Q in all problems and examples was either a given value or was obtained from the First Law relation. The flue gas leaves the boiler at 300oC all the water formed will be vapour. Complete combustion does NOT give carbon monoxide or sootCheck me out.
 Source: youtube.com
Source: youtube.com
Clean and stable combustion. The combustion reaction of methane is carried out even with an insufficient supply of oxygen. Not exactly the byproducts but simply the products of the reaction on a chemical point of view. On burning of natural gas ie combustion of methane leads to oxidation reaction and formation of carbon dioxide takes place along with the removal of water. Only exceptionally N F content excess09.
 Source: ohio.edu
Source: ohio.edu
The combustion reaction of methane is carried out even with an insufficient supply of oxygen. Governing EquationsNatural Gas and Biogas Combustion Modeling The equations used for natural gas biogas and natural gasbiogas mixture fuels combustion modeling 3 are based on the equations of conservation of. Chemically this process consists of a reaction between methane and oxygen. Complete Combustion Of Propane C3h8 Balanced Equation You. The balanced reaction is.
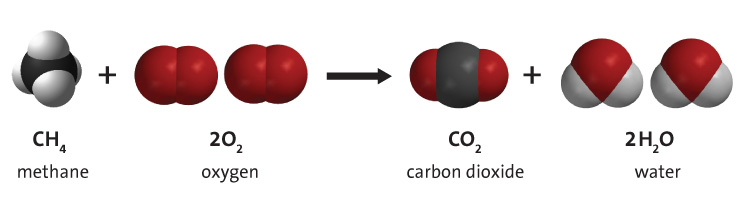 Source: energyeducation.ca
Source: energyeducation.ca
When this reaction takes place the result is carbon dioxide CO 2 water H 2 O and a great deal of energy. Table 1 shows values of H formation of several natural gas reactants and products. Calculate the heat of combustion of ethane as described in the equation C2H6g 3½O2g 2CO2g 3H2Ol given the heats of formation of ethane gas carbon dioxide gas and water liquid are 847 kJ mol-1 -3935 kJ mol-1 and 2858 kJ mol-1 respectively. The H comb of one mole of methane CH 4 at 29815 K is the heat of reaction between CH 4 and O 2 to form CO 2 g and H 2 Og according to Equation 1. Natural gas is the cleanest burning fossil fuel.
 Source: people.wou.edu
Source: people.wou.edu
Clean and stable combustion. Major groups of nitric compounds are. Environmental emission of carbon dioxide CO 2 when combustion fuels like coal oil natural gas LPG and bio energy. The Combustion of Methane. Loss -366 Total input 5832 5838 Stage 3 Primary Chamber Outlet Gas Stream Sensible SCC Inlet XCS air or Item lbhr Heat MMBtuh Gas temp O2 dry Total gas 102786 Solids 000 80.
 Source: youtube.com
Source: youtube.com
Interaction Of Methane With Oxygen Combustion Reaction Mel Chemistry. In addition a substantial amount of heat is also lost through the boiler wall and other uncounted sources. Natural gas is methane CH4 and what you call burning is a combustion it means. For residential and commercial space heating. Complete combustion does NOT give carbon monoxide or sootCheck me out.
 Source: people.wou.edu
Source: people.wou.edu
When this reaction takes place the result is carbon dioxide CO 2 water H 2 O and a great deal of energy. We would ASSUME there would be SOME incomplete combustion to give carbon monoxide and SOOT as well as some carbon dioxide the product of complete combustionand so let us write out the THREE oxidation reactions. The H comb of one mole of methane CH 4 at 29815 K is the heat of reaction between CH 4 and O 2 to form CO 2 g and H 2 Og according to Equation 1. Complete Combustion Of Propane C3h8 Balanced Equation You. 14 Natural Gas Combustion 141 General1-2 Natural gas is one of the major fuels used throughout the country.
 Source: ohio.edu
Source: ohio.edu
Not exactly the byproducts but simply the products of the reaction on a chemical point of view. Calculate the flue gas composition both dry and wet basis leaving a boiler. COMBUSTION AND FUELS FUEL NITROGEN IN GAS Natural gas practically doesnt have fuel nitrogen. COMBUSTION AND FUELS FUEL NITROGEN IN LIQUID FUELS Crude oil has fuel nitrogen in the range of 001 do 03 wt. Introduction - Up to this point the heat Q in all problems and examples was either a given value or was obtained from the First Law relation.
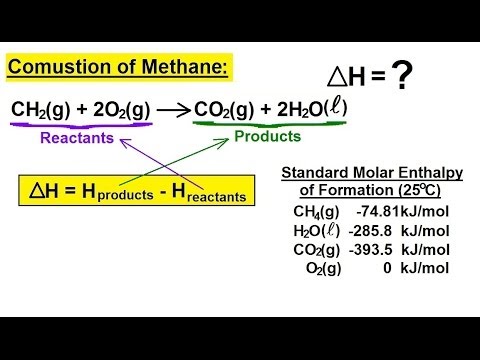 Source: youtube.com
Source: youtube.com
Coal or wood. The balanced reaction is. Natural gas consists of a high percentage of methane generally above. Respect to natural gas combustion. Equations 2 and 3 show the calculation for H reax ie.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title natural gas combustion equation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






