Your The element x has three naturally occurring isotopes images are available in this site. The element x has three naturally occurring isotopes are a topic that is being searched for and liked by netizens today. You can Find and Download the The element x has three naturally occurring isotopes files here. Download all royalty-free photos.
If you’re searching for the element x has three naturally occurring isotopes pictures information related to the the element x has three naturally occurring isotopes keyword, you have come to the right blog. Our site always provides you with hints for viewing the maximum quality video and picture content, please kindly search and find more enlightening video content and graphics that fit your interests.
The Element X Has Three Naturally Occurring Isotopes. The 83rd element bismuth was traditionally regarded as having the heaviest stable isotope bismuth-209 but in 2003 researchers in Orsay France measured the half-life of 209 Bi to be 19 10 19 years. There are actually three stable isotopes of neon with the unmentioned one being Ne-21. Mg-24 represents 790 of all magnesium atoms Mg-25 accounts for. 655 44Cv which has an atomic mass of.
 Solution An Unknown Element X Has Thre Chemistry From clutchprep.com
Solution An Unknown Element X Has Thre Chemistry From clutchprep.com
The abundance of the lightest isotope is 4238. The element X has three naturally occurring isotopes. 4 Calculate number of Ne-22 atoms. You can only solve for two variables at a time so the question will need to give you the percent abundances of all but two of the isotopes. Mg-24 represents 790 of all magnesium atoms Mg-25 accounts for. The average atomic mass of the element is __________ amu.
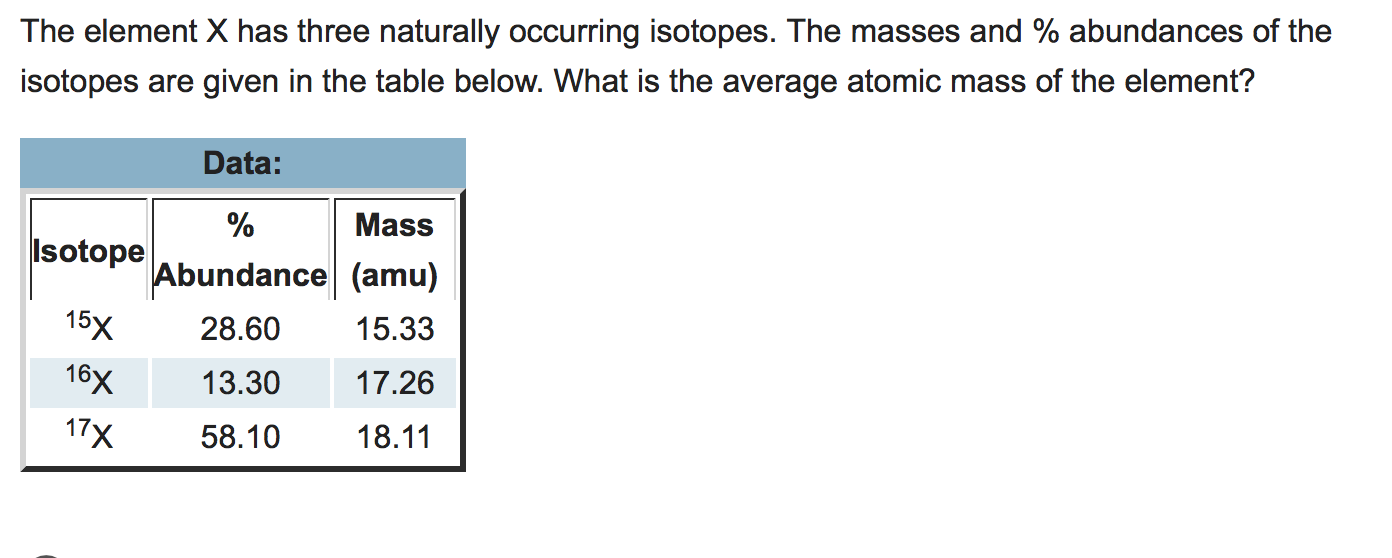
The masses amu and abundances of the isotopes are given in the table below.
Uranium has three common isotopes. 41 The element X has three naturally occurring isotopes. The masses amu and abundances of the isotopes are given in the table below. No other element has played as large a role as carbon on our planet. The average atomic mass of the element is amu. The element X has three naturally occurring isotopes.
 Source: chegg.com
Source: chegg.com
The average atomic mass of the element is ____amu. 4 Calculate number of Ne-22 atoms. The test question might round the isotope weights differently. The element X has three naturally occurring isotopes. The masses amu and abundances of the isotopes are given in the table below.
 Source: brainly.com
Source: brainly.com
What is the average mass of the element. 35Chlorine has two naturally occurring isotopes Cl 34. It is well known that the element magnesium has three naturally occurring isotopes. The abundance of the lightest isotope is 4238. Another 03 of the atoms are ceNe-21 which is an isotope of neon with 11 neutrons and a mass of 2099.
 Source: chegg.com
Source: chegg.com
Which of the three isotopes will exist in highest natural abundance. The percent abundance for Ne-21 is 02700. The masses of the three isotopes are 54335 59345 and 62349 The two have a percent abundance of 35528 and 38136 respectively. The relative abundance and atomic masses are 69. Copper is listed on the periodic table as having a relative atomic mass of 6355.
 Source: chegg.com
Source: chegg.com
There are actually three stable isotopes of neon with the unmentioned one being Ne-21. The average atomic mass of the element is __________ amu. The problem above is simplified. Which of the three isotopes will exist in highest natural abundance. Technetium and promethium atomic numbers 43 and 61 respectively and all the elements with an.

What is the average atomic mass of an element and how does it differ from the mass number. The mass number represents the protons plus the. An element has three naturally occurring isotopes with the following abundances and masses. Mg-24 represents 790 of all magnesium atoms Mg-25 accounts for. 749 Lithium-7 7016 amu 1-x 1 00749 09251 9251 6015x 70161-x 6941 x 07492 5.
 Source: chegg.com
Source: chegg.com
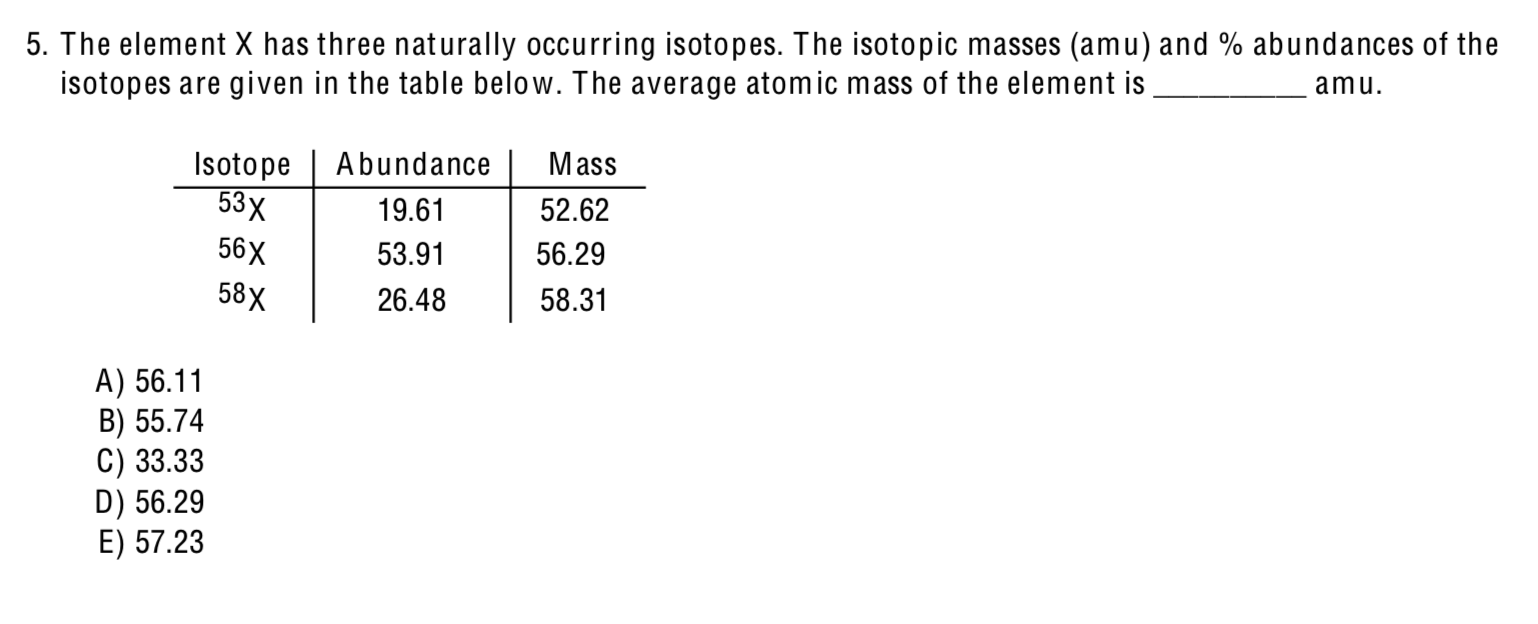
An imaginary element Atomic weight 937140 has three naturally-occurring isotopes with isotopic weights of 929469 932923 and 949030. If element X has three naturally occurring isotopes X-12 X13 An element X has been atomic mass of 1402 amu. Isotope 53x Abundance 9 1961 Massamu 5262 36x 5391 5629 158x 2648 5831 0 3333 5574 O 5723 5629 5611. 655 44Cv which has an atomic mass of. The average atomic mass of the element is ____amu.
 Source: oneclass.com
Source: oneclass.com
Element X has three naturally occurring isotopes. The masses amu and abundances of the isotopes are given in the table below. 15937 amu 3060 16279 amu 15. If element X has three naturally occurring isotopes X-12 X13 and X-14. Copper is listed on the periodic table as having a relative atomic mass of 6355.
 Source: chegg.com
Source: chegg.com
An imaginary element Atomic weight 937140 has three naturally-occurring isotopes with isotopic weights of 929469 932923 and 949030. Element X has three naturally occurring isotopes. The average atomic mass of the element is _____ amu. The masses amu and abundances of the isotopes are given in the table below. The problem above is simplified.
 Source: brainly.com
Source: brainly.com
An unknown element X has three naturally occurring isotopes. The average atomic mass of the element is amu. The masses amu and abundances of the isotopes are given in the table below. Abundancemass amu 789923985042 100024985837 110125982593Determine the molar mass of the element. 749 Lithium-7 7016 amu 1-x 1 00749 09251 9251 6015x 70161-x 6941 x 07492 5.
 Source: chegg.com
Source: chegg.com
Naturally occurring europium Eu consists of two isotopes with a mass of 151 and 153. The masses amu and abundances of the isotopes are given in the table below. Abundancemass amu 789923985042 100024985837 110125982593Determine the molar mass of the element. 41 The element X has three naturally occurring isotopes. An element has three naturally occurring isotopes with the following abundances and masses.
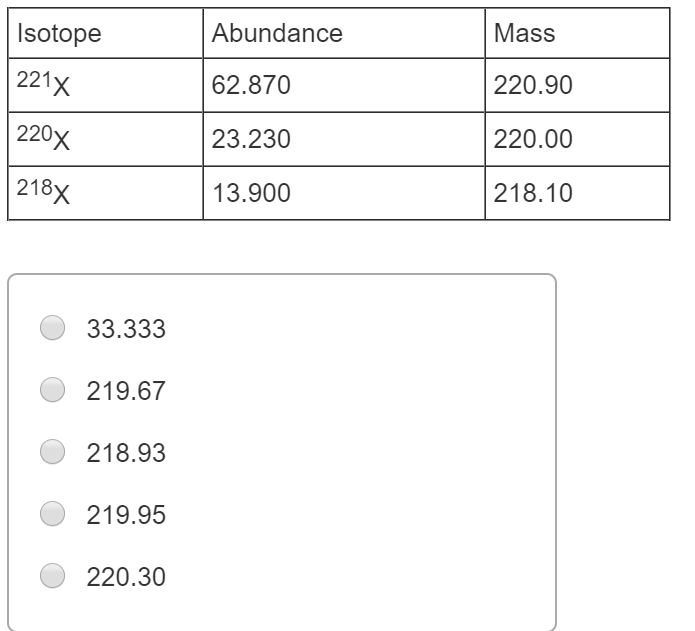 Source: chegg.com
Source: chegg.com
The isotopic masses and abundances are. The element X has three naturally occurring isotopes. 655 44Cv which has an atomic mass of. 41 The element X has three naturally occurring isotopes. An imaginary element Atomic weight 937140 has three naturally-occurring isotopes with isotopic weights of 929469 932923 and 949030.
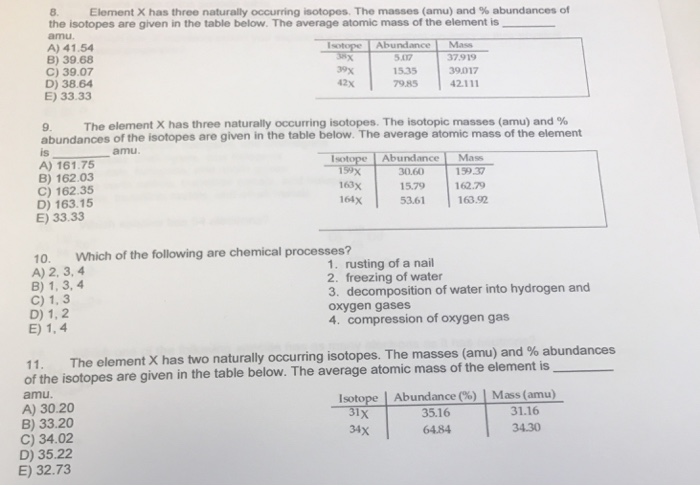
What is the average mass of the element. There are actually three stable isotopes of neon with the unmentioned one being Ne-21. The problem above is simplified. The element X has three naturally occurring isotopes. The atomic mass of an element is the average of all of the masses of the naturally occurring isotopes of that element.
 Source: chegg.com
Source: chegg.com
79 and 16392 amu 5361. The average atomic mass of the element is __________ amu. Neon has three naturally occurring isotopes. Since the sum of the isotopic abundance percentages is equal to 1 100 the formula is. You may receive a question involving more than two isotopes of an element.
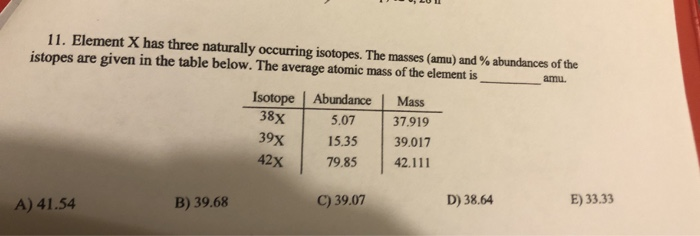 Source: chegg.com
Source: chegg.com
It is well known that the element magnesium has three naturally occurring isotopes. Element X has three naturally occurring isotopes. An element has three naturally occurring isotopes with the following abundances and masses. The element X has three naturally occurring isotopes. The problem above is simplified.
 Source: chegg.com
Source: chegg.com
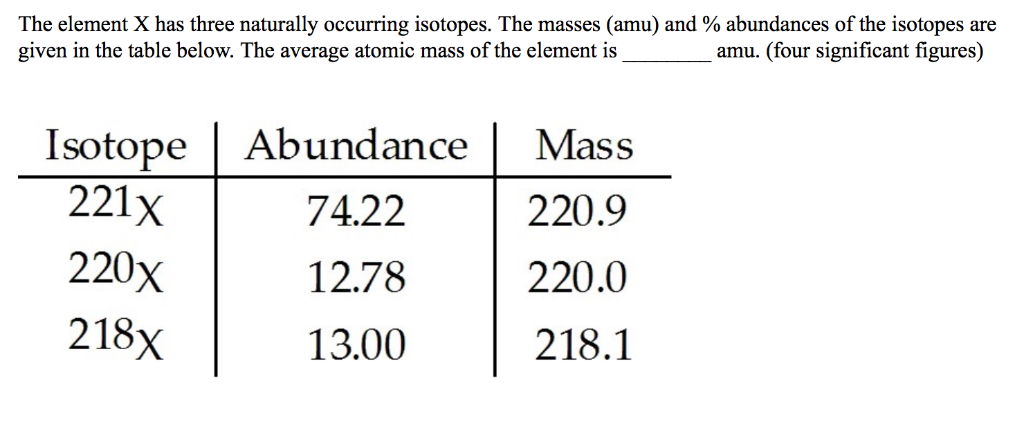
655 44Cv which has an atomic mass of. The test question might round the isotope weights differently. The isotopic masses and abundances are. What is the average atomic mass of an element and how does it differ from the mass number. Isotope Abundance Mass 221X 7422 2209 220X 1278 2200 218X 1300 2181.
 Source: chegg.com
Source: chegg.com
The average atomic mass of the element is __________ amu. An element has three naturally occurring isotopes with the following abundances and masses. Abundancemass amu 789923985042 100024985837 110125982593Determine the molar mass of the element. Which of the three isotopes will exist in highest natural abundance. Here is a neon problem involving all three isotopes.
 Source: chegg.com
Source: chegg.com
The test question might round the isotope weights differently. The percent abundance for Ne-21 is 02700. The test question might round the isotope weights differently. 3745 x 10 23 times 0095 3558 x 10 22. Abundancemass amu 789923985042 100024985837 110125982593Determine the molar mass of the element.
 Source: clutchprep.com
Source: clutchprep.com
Though the element has as many as 15 isotopes only three are naturally occurring while the rest are artificially transmuted elements ephemeral in nature lasting from a few nanoseconds to a few minutes. The average atomic mass of the element is __________ amu. The average atomic mass of the element is _____ amu. You may receive a question involving more than two isotopes of an element. The element X has three naturally occurring isotopes.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title the element x has three naturally occurring isotopes by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






